Emerging Contaminant Spotlight – 1,4 Dioxane
Emerging Contaminant Spotlight – 1,4 Dioxane
The emerging contaminant 1,4 dioxane commonly referred to as “1,4 d” is an industrial compound that was first synthesized in the 1950’s. It is estimated that 90% of 1,4 dioxane use was as a stabilizer in chlorinated solvents until this usage was eliminated sometime in the mid-to-late 1990’s. In 1990, approximately 10.5 to 18 million (M) pounds of 1,4 dioxane was produced in the United States. According to USEPA Toxic Release Inventory for 1996, approximately 1M pounds of 1,4 dioxane was released into the environment.
What is the Contaminant Source?
1,4 dioxane is also used in textile manufacturing, paint strippers, the development of dyes, grease, varnishes and waxes. It can also be found as an impurity in aircraft de-icing fluids and some personal care products such as shampoo,makeup, deodorants and trace amounts can be produced in the production of pharmaceuticals. Recently, 1,4 dioxanehas been discovered in food packaging adhesives, food supplements and food crops sprayed with pesticides containing 1,4 dioxane. It is commonly found at polyethylene terephthalate (PET) plastic manufacturing facilities.
Although, 1,4 dioxane has been around for over 65 years, its emerging status derives from awareness that this compound has been discovered to be more pervasive and widespread than previously thought. Commonly associated with chlorinated solvent (trichloroethene (TCE) and trichloroethane (TCA)) release sites from its predominant past usage, 1,4 dioxane has been discovered in Public Water Supply Systems (PWSS), wastewater treatment plant (WWTP) effluent streams and closed landfills. USEPA has determined that 1,4 dioxane is likely to be carcinogenic with inhalation and ingestion as the most likely routes of exposure.
Where is it Located?
USEPA has indicated that there are a substantial number of chlorinated solvent release sites that have not been tested for 1,4 dioxane according to research as it is not part of the typical volatile organic compound (VOC) suite of analytes. Further complicating 1,4 dioxane site characterization is the variation in laboratory analysis using different testing methods. These methods all focus on low level detection and have presented order of magnitude differences in analytical results depending on the methods used.
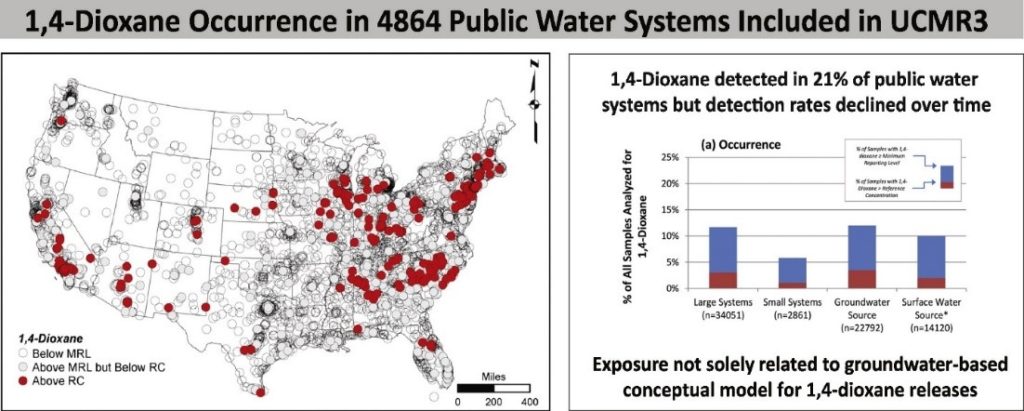
As can be seen from the graphics below, according to USEPA, through the Unregulated Contaminant Monitoring Rule 3 (UCMR 3) sampling completed 2013-2015, 341 out of 4915 (6.9%) PWSS had 1,4 dioxane concentrations exceeding the reference concentration of 0.35 parts per billion (currently the USEPA health advisory concentration). UCMR 4, scheduled for testing approximately 6,000 public water supply system (PWSS) affected by both surface and groundwater in 2018-2020, will include 1,4 dioxane. Interesting to note that although 21% of PWSS had 1,4 dioxane detection, the detection rate has declined over time.
1,4 dioxane is readily miscible in water (hydrophilic) and does not generally sorb to soil. Resultant release to ground or surface water, or comingled plumes containing 1,4 dioxane can be more extensive than might be expected due to higher contaminant velocity than typical solvent plumes. It is relatively resistant to biodegradation; however, recent research has shown that under certain conditions biodegradation can occur.
What is the MCL for 1,4 Dioxane
Adding to the inconsistency surrounding 1,4 dioxane
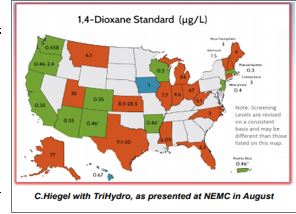 is the regulatory framework. USEPA has notestablished a Maximum Contaminant Level (MCL)for 1,4 dioxane. The graphic presented below showscurrent state health advisory levels. USEPA has established a 0.35 ug/L for a 10-6 cancer risk in drinking water. A reportable quantity of 100 pounds has been established by EPA under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).
is the regulatory framework. USEPA has notestablished a Maximum Contaminant Level (MCL)for 1,4 dioxane. The graphic presented below showscurrent state health advisory levels. USEPA has established a 0.35 ug/L for a 10-6 cancer risk in drinking water. A reportable quantity of 100 pounds has been established by EPA under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).
Where are the Opportunities to provide Consulting Services?
Environmental opportunities related to 1,4 dioxane include sampling previously non-sampled chlorinated release sites, site characterization, fate and transport modeling and remediation. The development of a robust Conceptual Site Model will be important to effective remedial strategy development. Bioremediation, phytoremediation, chemical oxidation and electrical resistive heating have all been effectively demonstrated under certain geologic, hydrogeologic and geochemical conditions. Pump and treat with ex-situ treatment using fixed film, biological moving bed treatment has also been successful.
This article generously provided by GBA’s Environmental Business Council to benefit all geoprofessionals providing environmental consulting services.
(all images courtesy of Internet)